1. The task force recommends patients with any of the following undergo a genetic evaluation: 2 or more lifetime hamartomatous polyps, a family history of hamartomatous polyps, or a cancer associated with a hamartomatous polyposis syndrome in first or second-degree relatives. Genetic testing (if indicated) should be performed using a multigene panel test.
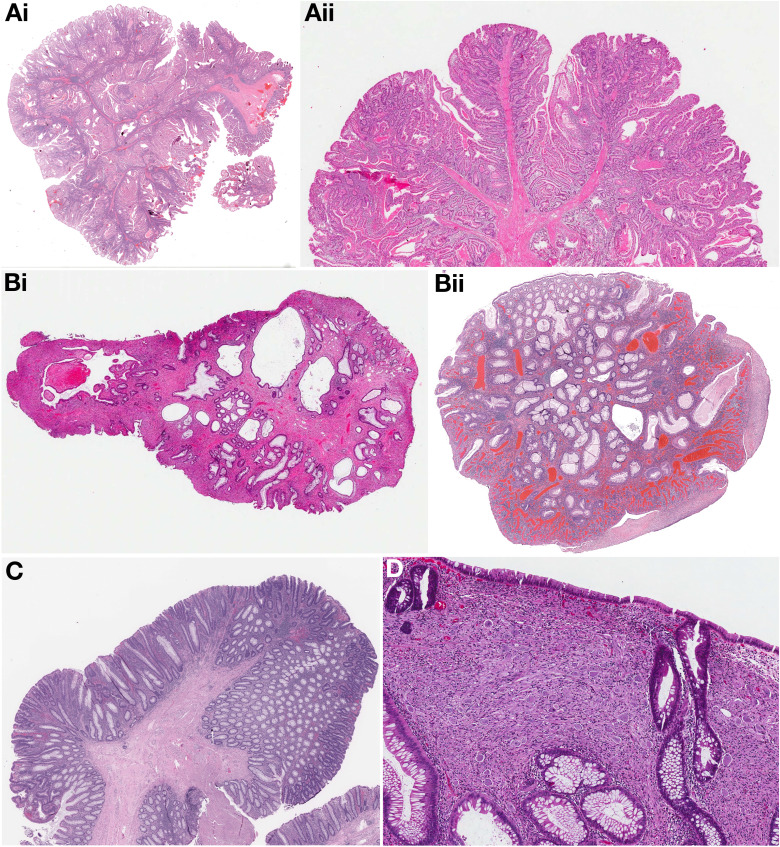
2. The task force recommends genetic evaluation for any individual with the following: 1) 2 or more histologically confirmed Peutz-Jeghers polyps, 2) any number of Peutz-Jeghers polyps in an individual who has a family history of Peutz-Jeghers syndrome in a first-degree relative, 3) characteristic mucocutaneous pigmentation in a person with a family history of Peutz-Jeghers syndrome, 4) any number of Peutz-Jeghers polyps in a person with the characteristic mucocutaneous pigmentation of Peutz-Jeghers syndrome.
3. Patients with Peutz-Jeghers syndrome are at increased risk for cancer in multiple organs including cancer of the breast, small bowel, colon, stomach, pancreas, ovaries, testes, and lungs.
Given this risk, we recommend a multidisciplinary approach to cancer surveillance in these organs.
4. The task force recommends that baseline small bowel surveillance using video capsule endoscopy or magnetic resonance enterography be performed between ages 8-10 years or earlier if the patient is symptomatic. If no polyps are found at the initial examination, surveillance should resume at age 18. Because of the risk of small bowel intussusception, small bowel surveillance in adulthood is recommended to continue throughout life every 2-3 years.
5. The task force suggests a baseline upper gastrointestinal endoscopy between the ages of 8 and 10 years, which could be performed at the time of capsule placement for small bowel surveillance or if polyps are identified on magnetic resonance enterography. Although the initiation age for colonoscopy remains uncertain, we also suggest initiation of colonoscopy at same time as esophagogastroduodenoscopy. In those in whom characteristic polyps are detected, both colonoscopy and esophagogastroduodenoscopy should be repeated every 2-3 years. In those in whom there are no Peutz-Jeghers polyps at baseline, surveillance is repeated at age 18 years, or sooner should symptoms arise, and then every 3 years.
6. The task force recommends polypectomy of small bowel polyps that are symptomatic or ≥10 mm to prevent intussusception and other complications, such as bleeding.
7. The task force suggests annual pancreatic cancer surveillance with either magnetic resonance cholangiopancreatography or endoscopic ultrasound starting at age 35 years.
8. The task force recommends genetic evaluation for any individual with 1) 5 or more juvenile polyps of the colon or rectum; or 2) 2 or more juvenile polyps in other parts of the gastrointestinal tract; or (3) any number of juvenile polyps and 1 or more first-degree relatives with juvenile polyposis syndrome.
9. Juvenile polyposis syndrome patients are at increased risk for cancer in multiple organs including cancer of the colon and stomach. Given this risk, the task force recommends patients with juvenile polyposis syndrome undergo surveillance of the colon and stomach.
10. The task force suggests initiating colonoscopic and upper endoscopic surveillance at age 12-15 years, or earlier if symptomatic. Surveillance should be repeated every 1-3 years depending on polyp burden.
11. The task force suggests patients with SMAD4 pathogenic variants be clinically evaluated for HHT at the time of the diagnosis, including screening for and appropriate management of cerebral and pulmonary AVMs.
12. The task force recommends individuals with multiple gastrointestinal hamartomas or ganglioneuromas undergo genetic evaluation for Cowden’s syndrome and related conditions.
13. In PTEN hamartoma tumor syndrome, patients are at increased risk for cancer in multiple organs, including cancer of the breast, thyroid, kidney, uterus, colon, and skin. Given this risk, the task force recommends a multi-disciplinary approach to cancer surveillance in these organs.
14. The task force suggests colonoscopy surveillance to begin at age 35 years (or 10 years younger than age of any relative with colorectal cancer), repeated at intervals no greater than 5 years, depending on polyp burden.