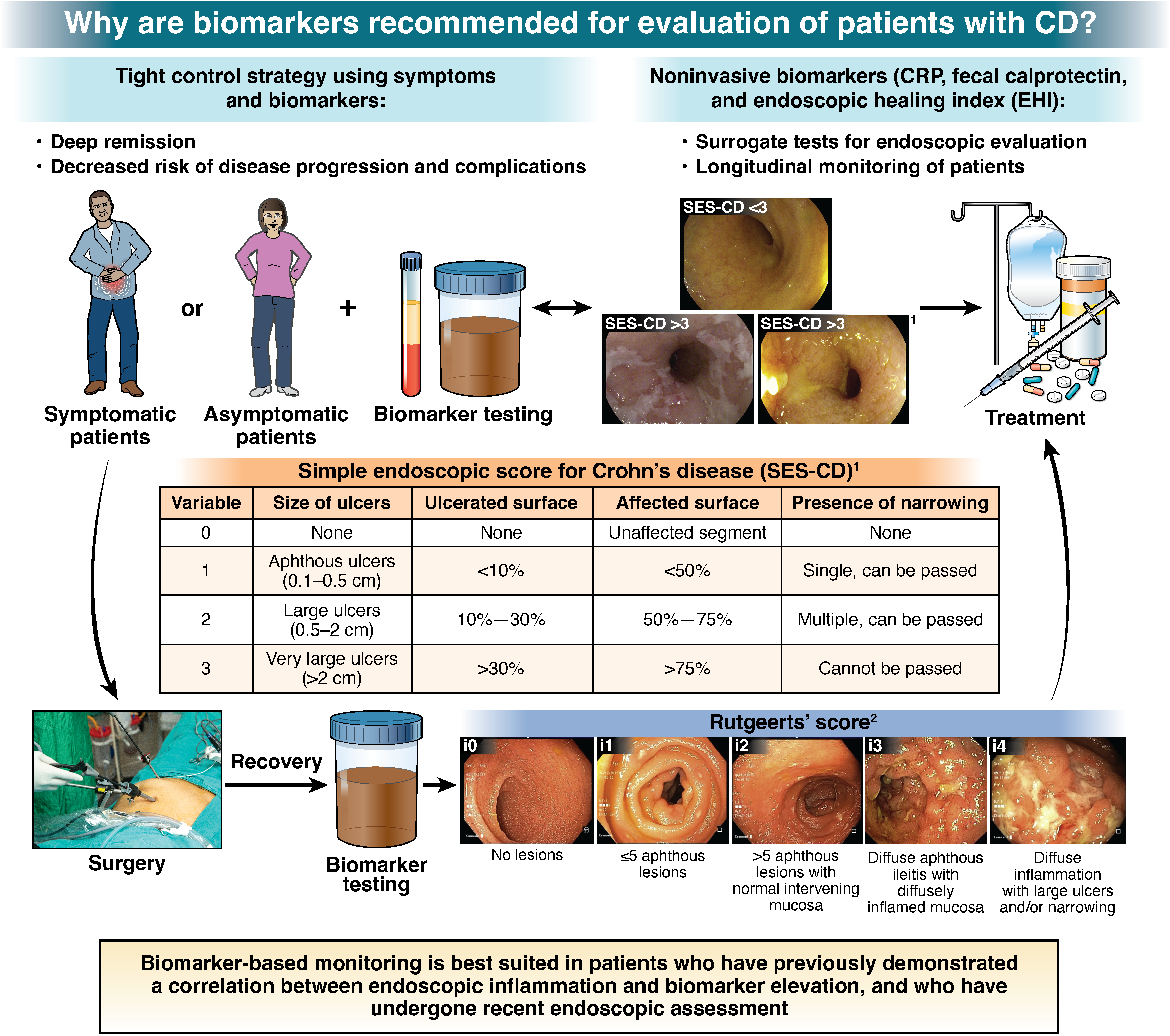
1. In patients with CD in symptomatic remission, the AGA suggests a monitoring strategy that combines biomarkers and symptoms, rather than relying on symptoms alone.
2. In patients with CD in symptomatic remission with recent confirmation of endoscopic remission (without any change in clinical status, on stable therapy) the AGA suggests using fecal calprotectin <150 μg/g and/or CRP <5 mg/L (or below cut-off for normal range for the laboratory) to rule out active inflammation, and avoid routine endoscopic assessment of disease activity.
3. In patients with CD in symptomatic remission without recent confirmation of endoscopic remission, the AGA suggests endoscopic evaluation to rule out active inflammation, rather than relying solely on fecal calprotectin or CRP.
4. In patients with CD in symptomatic remission, with elevated biomarkers of inflammation (fecal calprotectin >150 µg/g, CRP >5 mg/L), the AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
5. In patients with symptomatically active CD, the AGA suggests a biomarker-based assessment and treatment adjustment strategy, rather than relying on symptoms alone.
6. In patients with CD with mild symptoms and elevated biomarkers of inflammation (fecal calprotectin >150 µg/g, CRP >5 mg/L), the AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
7. In patients with CD with mild symptoms and normal biomarkers of inflammation (fecal calprotectin <150 µg/g, CRP <5 mg/L), the AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
8. In patients with CD with moderate to severe symptoms, the AGA suggests using fecal calprotectin >150 µg/g or CRP >5 mg/L, to rule in active inflammation and inform treatment adjustment and avoid routine endoscopic assessment of disease activity.
9. In patients with CD with moderate to severe symptoms with normal biomarkers of inflammation (fecal calprotectin <150 µg/g, CRP <5 mg/L), the AGA suggests endoscopic assessment of disease activity rather than empiric treatment adjustment.
10. In asymptomatic patients with CD following surgically-induced remission within the past 12 months who are at low risk of post-operative recurrence or who have one or more risk factors for recurrence but are on post-operative pharmacologic prophylaxis, , the AGA suggests using fecal calprotectin <50 µg/g, to avoid routine endoscopic assessment of disease activity.
11. In asymptomatic patients with CD following surgically-induced remission within the past 12 months, who are at high baseline risk of recurrence AND are not receiving post-operative pharmacologic prophylaxis, the AGA suggests endoscopic evaluation, rather than relying solely on biomarkers, for assessing endoscopic recurrence.
12. In patients with CD, the AGA suggests neither in favor of nor against the use of endoscopic healing index (EHI, Monitr®) for monitoring inflammation and treatment decisions.
13. In patients with CD, the AGA makes no recommendation in favor of, or against, a biomarker-based monitoring strategy over an endoscopy-based monitoring strategy to improve long-term outcomes.